Getting your custom retail packaging wrong can result in costly regulatory fines, product recalls, and lost customer trust. With complex labeling requirements varying by region and product type, businesses often struggle to ensure compliance while maintaining attractive packaging design.
Custom retail packaging must include essential information such as product identity, net quantity, manufacturer details, safety warnings, and regulatory compliance statements. The specific requirements depend on your product category, target market, and applicable regulations like the Fair Packaging and Labeling Act (FPLA) or FDA guidelines.
Understanding these requirements is crucial for any business launching products in retail markets worldwide.
Table of Contents
ToggleWhat Are the Core Legal Requirements for Retail Packaging Labels?
The foundation of retail packaging compliance starts with understanding mandatory federal requirements that apply to most consumer products under established regulatory frameworks.
All retail packaging must display three fundamental elements mandated by the Fair Packaging and Labeling Act (FPLA): statement of identity, net quantity of contents, and name and place of business of the manufacturer, packer, or distributor. These requirements are enforced by the FDA for foods, drugs, cosmetics, and medical devices, while the FTC oversees other consumer commodities.

Federal Regulatory Framework
The Fair Packaging and Labeling Act serves as the primary legislation governing consumer commodity labeling, designed to facilitate value comparisons and prevent unfair or deceptive packaging practices. This comprehensive law applies to most household consumer commodities and works in conjunction with the Federal Food, Drug, and Cosmetic Act (FD&C Act) to provide regulatory authority.
Statement of Identity Requirements
- Must be a principal feature positioned prominently on the package
- Should be parallel to the package base using large, bold type
- Must be at least half the size of the largest font on the package
- Cannot be misleading or deceptive about the product’s nature
Net Quantity Declaration Standards
- Must appear in the lower third of the principal display panel
- Requires both avoirdupois and metric units (e.g., “Net Wt 15 oz (425 g)”)
- Type size determined by total PDP area according to FDA specifications
- Must state weight for solids, volume for liquids, or count where appropriate
Manufacturer Identification
- Complete name and place of business required
- Qualifying phrases like “Manufactured for” or “Distributed by” when applicable
- Must be clearly visible and easily readable
- Contact information for customer inquiries
| Requirement | Regulatory Authority | Placement | Format |
|---|---|---|---|
| Product Identity | FDA/FTC | Principal Display Panel | Prominent, large type |
| Net Quantity | FDA/FTC | Lower third of PDP | Dual units required |
| Manufacturer Info | FDA/FTC | Information Panel | Complete address |
How Do Safety and Warning Requirements Affect Your Packaging Design?
Safety warnings and hazard communications are regulated by multiple agencies and can significantly impact your packaging layout, requiring careful integration with design elements.
Safety requirements are overseen by the Consumer Product Safety Commission (CPSC) for consumer products, with specific mandates including tracking labels for children’s products, small parts warnings for items intended for ages 3-6, and hazardous substance labeling under the Federal Hazardous Substances Act.

Product-Specific Safety Mandates
The regulatory landscape for safety warnings varies dramatically based on product category and associated hazards, with each requiring specific compliance measures.
Children’s Product Requirements
- Tracking labels mandatory for all children’s products under CPSC oversight
- Small parts warnings required for products intended for children aged 3-6
- Child-resistant packaging under the Poison Prevention Packaging Act (PPPA) for hazardous products
- 85% of children under 5 cannot open packages within 5 minutes
- 80% of adults aged 50-70 can open and properly close within same timeframe
Hazardous Materials Compliance
- Weather-resistant, durable labels meeting 49 CFR regulations
- Specific colors, codes, and pictograms for hazard identification
- Markings in English on contrasting backgrounds
- Positioned away from other markings to maintain effectiveness
Suffocation Prevention
- Warning labels required on flexible film packaging 1 mil or less in thickness
- Must include clearly marked “WARNING” or “CAUTION” language
- Risk statements about suffocation dangers
- Instructions to keep bags away from children
When designing packaging for regulated categories, safety information must be seamlessly integrated without compromising brand aesthetics. At Acreet, we specialize in creating compliant packaging designs that maintain visual appeal while meeting all CPSC and FDA safety requirements.
What Information Must Be Displayed on the Principal Display Panel?
The principal display panel (PDP) represents the most critical packaging real estate, with specific sizing, positioning, and content requirements that determine regulatory compliance.
The principal display panel must accommodate all mandatory information with clarity and conspicuousness. For rectangular packages, the PDP equals height times width of the primary display side, while for cylindrical containers, it represents 40% of the height times circumference.
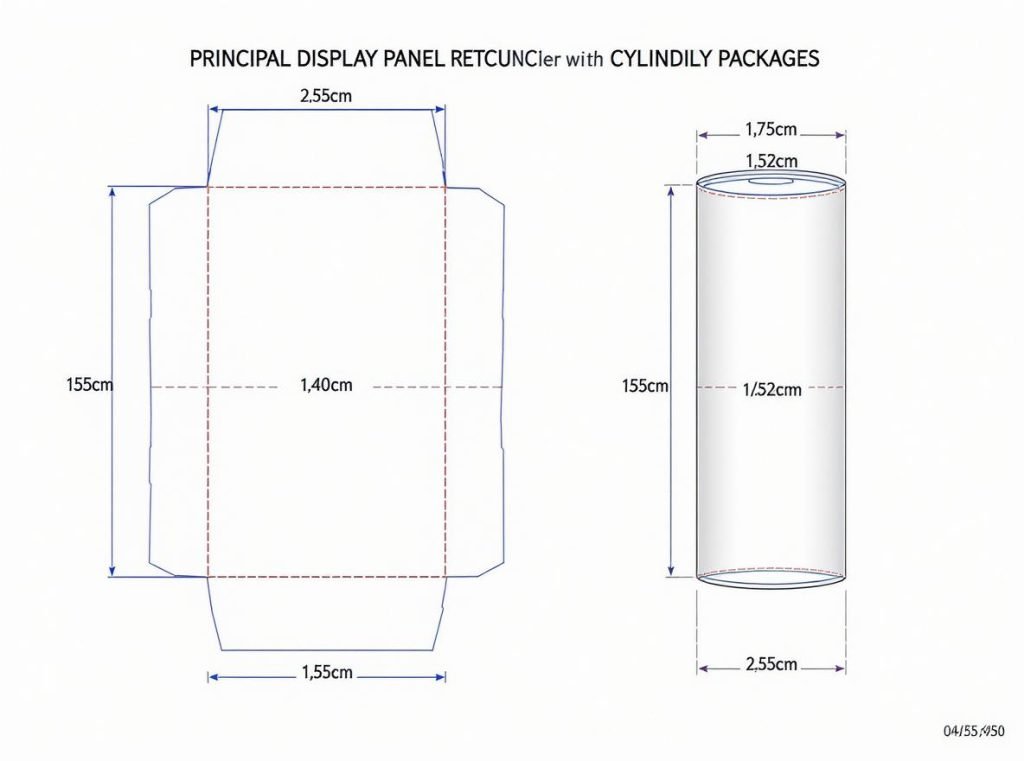
PDP Sizing and Content Standards
Federal regulations establish precise requirements for PDP dimensions and information hierarchy to ensure consumer accessibility and regulatory compliance.
Dimensional Requirements:
- Rectangular packages: Height × width of customer-facing side
- Cylindrical containers: 40% of height × circumference
- Must be large enough to accommodate required information without crowding
- Cannot obscure designs or create visual confusion
Mandatory PDP Content Hierarchy:
- Statement of identity as principal feature in middle of label
- Net quantity declaration in lower 30% of PDP
- Required safety warnings prominently displayed
- All text in contrasting colors to background
Legibility Standards:
- Clear, easy-to-read fonts in contrasting colors
- Statement of identity at least half the size of largest package font
- Information duplicated on alternate PDPs where they exist
- Type size determined by total PDP area per FDA specifications
| Package Type | PDP Calculation | Required Elements | Positioning |
|---|---|---|---|
| Rectangular | Height × Width | Identity, quantity, warnings | Specific hierarchy |
| Cylindrical | 40% × (Height × Circumference) | Same requirements | Adapted to shape |
| Flexible | Customer-facing area | All mandatory info | Visible during display |
How Do International Market Requirements Affect Your Packaging?
Expanding into global markets introduces complex regulatory layers, with each region maintaining distinct labeling requirements that must be addressed in packaging design strategies.
International markets require region-specific compliance including CE marking for EU products, multi-language labeling, sustainability mandates, and country-specific regulatory statements. The EU has implemented comprehensive packaging and waste regulations with mandatory recycled content targets and “forever chemicals” restrictions.
European Union Compliance Framework
The EU maintains some of the world’s most stringent packaging regulations, with comprehensive requirements affecting both compliance and sustainability.
CE Marking Requirements:
- Mandatory for products covered by New Approach Directives
- Indicates compliance with EU health, safety, and environmental requirements
- Must include manufacturer’s name and address
- European Authorized Representative information where applicable
Sustainability Mandates:
- Packaging reduction targets: 5% by 2030, 10% by 2035, 15% by 2040
- Minimum recycled content requirements up to 65% for single-use plastic bottles by 2040
- Bans on “forever chemicals” (PFAS) in food contact packaging above certain thresholds
- Material composition and recycled content labeling for consumer sorting
Packaging and Waste Directive:
- All packaging must be recyclable with minimized harmful substances
- New restrictions on single-use plastic packaging effective January 1, 2030
- Covers pre-packed fruits and vegetables under 1.5 kg
- Various food service items included in restrictions
Multi-Market Considerations:
- Language-specific labeling requirements varying by country
- Regional safety standard compliance
- Import certification numbers for Asian markets
- Cultural considerations for colors and symbols
When planning international expansion, working with experienced packaging partners becomes essential. At Acreet, we help businesses navigate complex international compliance requirements while creating packaging solutions that work effectively across multiple markets.
What Are the Specific Requirements for Food and Cosmetic Packaging?
Food and cosmetic products face the most stringent labeling requirements, with detailed nutritional information and ingredient disclosures that significantly impact packaging design and layout strategies.
Food packaging must include FDA-compliant Nutrition Facts labels with updated requirements including added sugars declarations, revised Daily Values, and enhanced calorie prominence. Cosmetic packaging requires ingredient lists in descending order of predominance, manufacturer information, and product identity statements.

Food Product Regulatory Requirements
Food packaging compliance involves multiple FDA regulations with specific formatting and content requirements that must be precisely followed.
Nutrition Facts Label Standards:
- Required for most packaged foods with updated format requirements
- Added sugars declarations and revised Daily Values mandatory
- Enhanced calorie prominence with larger, bolder font
- Cannot be placed on package bottoms unless visible during normal retail display
Allergen Disclosure Under FALCPA:
- Clear identification of eight major allergens required
- Must specify milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, and soybeans
- Can appear in ingredient list or separate “Contains” statement
- Specific type of tree nut, fish, or shellfish must be identified
Bioengineered Food Labeling:
- Mandatory disclosure since 2022 for genetically modified ingredients
- Must use term “bioengineered food” through labels, QR codes, or text messages
- Applies to foods containing detectable genetic material modified through laboratory techniques
- Various disclosure methods acceptable based on package size and format
Cosmetic Product Specifications
Cosmetic labeling operates under both the FD&C Act and FPLA, requiring specific information placement and formatting standards.
Required Information Elements:
- Product identity prominently featured on principal display panel
- Net quantity of contents with appropriate measurement units
- Ingredient list in descending order of predominance on information panel
- Manufacturer information with complete business address
Ingredient Disclosure Standards:
- All ingredients listed using established nomenclature
- Fragrances and flavors may be listed simply as “fragrance” or “flavor”
- Warning statements required for certain cosmetics as prescribed by regulation
- Information panel typically positioned to the right of PDP
How Can You Ensure Compliance While Maintaining Brand Appeal?
Balancing regulatory compliance with attractive packaging design requires strategic planning and expert knowledge of both legal requirements and design principles to create effective brand communication.
Successful compliance packaging integrates required information seamlessly into overall design aesthetic through strategic typography, color usage, and layout planning. This involves establishing clear brand guidelines with specific color codes, font families, and logo placement rules while accommodating mandatory information.

Design Integration Strategies
Modern packaging design treats compliance information as integral design elements rather than obstacles to creativity, requiring systematic approach to visual hierarchy.
Visual Hierarchy Development:
- Strategic use of contrasting colors and font sizes
- Positioning that guides consumer attention while ensuring compliance prominence
- Integration of required information into natural reading flow
- Balance between regulatory requirements and brand identity elements
Typography and Color Strategy:
- Brand-consistent font families that meet legibility requirements
- Color selection that satisfies contrast requirements while supporting brand identity
- Size relationships that emphasize required information appropriately
- Consistency across product lines and package formats
Material Selection Considerations:
- FSC-certified recyclable cardboard for sustainability compliance
- Biodegradable plastics meeting environmental regulations
- Materials with appropriate barrier properties for product protection
- Sustainable options that align with brand values and regulatory requirements
Implementation Best Practices:
- Cross-functional collaboration between design, regulatory, and manufacturing teams
- Regular review of applicable regulations and industry best practices
- Quality assurance systems integrated throughout production process
- Continuous monitoring of evolving regulatory landscape
Working with experienced packaging manufacturers who understand both regulatory requirements and design principles can save significant time and costs. Our team at Acreet combines deep regulatory expertise with creative design capabilities to deliver packaging that meets all compliance requirements while strengthening brand presence and consumer appeal.
What Are Common Compliance Mistakes to Avoid?
Understanding frequent compliance errors helps businesses avoid costly mistakes and regulatory issues that can delay product launches, require expensive recalls, or result in regulatory action.
Common mistakes include inadequate labeling with missing required information, insufficient product protection, failure to stay current with changing regulations, and poor documentation practices. These errors can result in FDA warnings, FTC actions, customs delays, or product recalls.

Frequent Compliance Pitfalls
Many businesses make predictable mistakes that can be easily avoided with proper planning, regulatory expertise, and systematic quality control measures.
Labeling and Information Errors:
- Missing required information elements on principal display panel
- Incorrect positioning of mandatory text and warnings
- Illegible text due to poor contrast or insufficient font size
- Print errors and typos requiring costly corrections or recalls
Product Protection Failures:
- Insufficient product protection for specific storage conditions
- Poor sealing or inappropriate material selection
- Inadequate cushioning for fragile products
- Failure to protect from contamination or environmental factors
Regulatory Oversight Issues:
- Failure to stay current with changing regulations
- Overlooking new requirements such as updated nutrition labeling rules
- Missing emerging sustainability mandates or international standard changes
- Inadequate regulatory research during product development
Documentation and Quality Control:
- Inadequate testing of packaging before shipment
- Poor record-keeping supporting nutrient declarations and safety testing
- Insufficient documentation for regulatory compliance verification
- Lack of systematic inspection protocols and quality assurance
Automation and System Failures:
- Over-reliance on automated compliance systems without proper oversight
- Inadequate testing of new packaging designs and formats
- Failure to maintain traceability throughout production process
- Poor integration between design, production, and quality control systems
| Mistake Category | Potential Impact | Prevention Strategy |
|---|---|---|
| Labeling Errors | Regulatory warnings, recalls | Professional design review |
| Protection Issues | Product damage, liability | Material testing and validation |
| Regulatory Gaps | Compliance violations, fines | Continuous monitoring system |
| Documentation | Regulatory action, delays | Systematic record-keeping |
Summary
Custom retail packaging must include essential information mandated by the Fair Packaging and Labeling Act: product identity, net quantity, and manufacturer details. Additional requirements vary by product category, with food packaging requiring Nutrition Facts labels and allergen disclosures, while cosmetic products need ingredient lists and safety warnings. International markets add complexity with CE marking, sustainability mandates, and multi-language requirements.

Ready to ensure your custom packaging meets all regulatory requirements while maintaining strong brand appeal? Contact Acreet today for expert packaging design and manufacturing services that guarantee compliance across all your target markets. Our experienced team combines deep regulatory knowledge with creative design capabilities to help you navigate complex requirements while creating packaging that drives sales and builds customer trust.


